The Brussels-Capital Region, a mainstay in promoting
the 3Rs principle and non-animal methods
In 2010, the European Union promulgated Directive 2010/63/EU for the protection of live vertebrate animals intended for animal experimentation and other scientific purposes. Belgian legislation partially transposed the Directive in the Royal Decree of 29 May 2013. That Decree includes rules regarding the housing of laboratory animals, the competent persons/authorities for the protection and welfare of laboratory animals and the implementation of the 3Rs principle in Belgium.
As a result of the sixth state reform that took place in 2014, the three Belgian regions, namely Flanders, the Brussels-Capital Region and Wallonia, were given more autonomy and some powers, such as Animal Welfare, were shifted from the federal level to the regional level. As a result, the three separate regions are responsible for monitoring compliance with the regulations in the Royal Decree on the protection of laboratory animals.
The Brussels-Capital Region has since then supported several initiatives by the VUB to reduce the number of laboratory animals and develop non-animal methods. Key actions have included the creation of the Innovation Centre 3Rs (IC-3Rs) and the RE-Place database. Read more about the RE-Place project, based on a collaboration between the VUB and Sciensano and jointly financially supported by the Flemish and Brussels governments, on their site www.re-place.be.
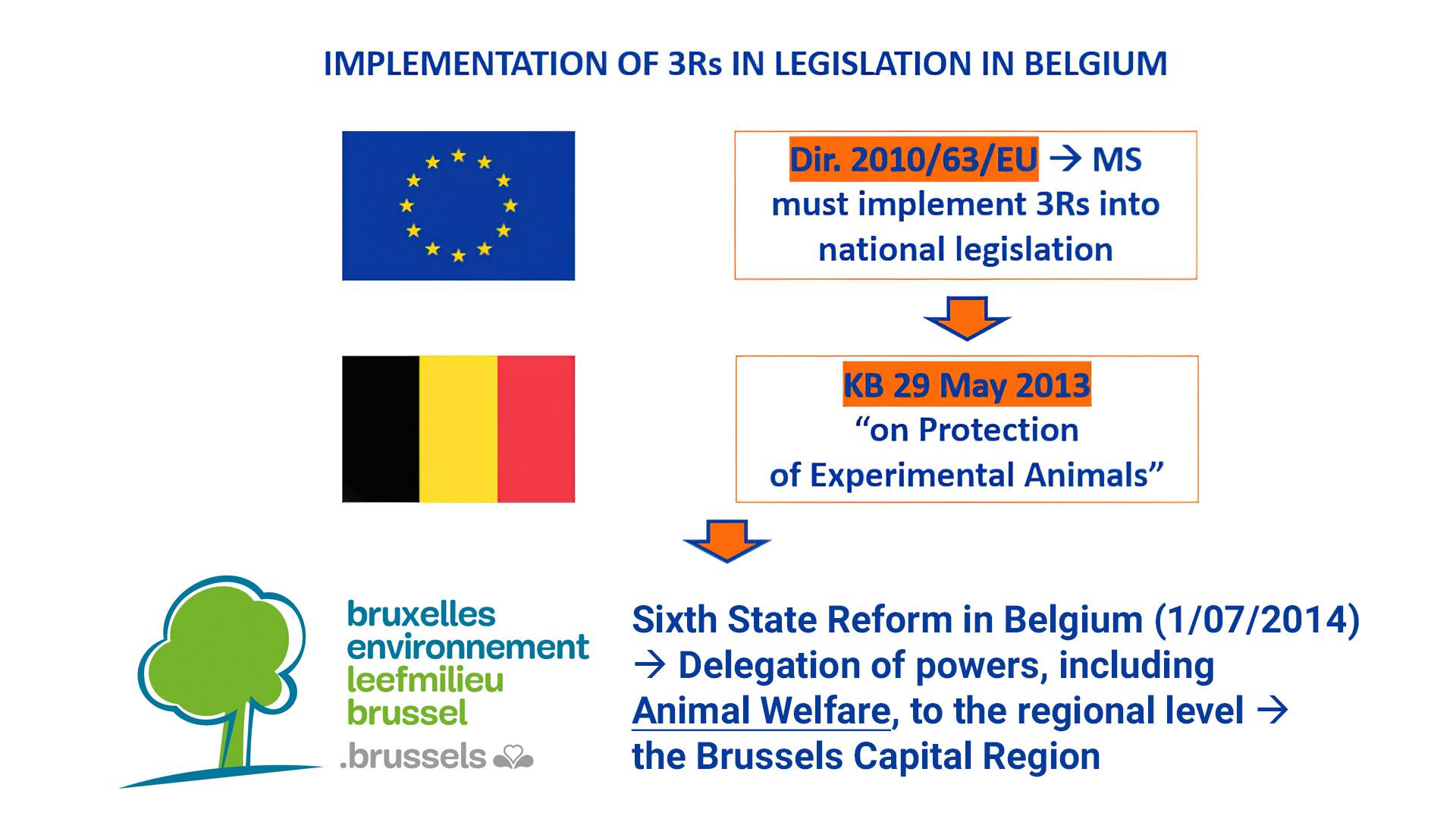
KB: Koninklijk Besluit (Royal Decree) Dir: Directive MS: Member states
How does the Brussels Capital Region support IC-3Rs?

The Brussels-Capital Region supports IC-3Rs' efforts to promote the 3Rs principle and especially non-animal methods in biomedical research. Brussels Environment provides financial support for doctoral theses, annual symposia, activities of the VUB Ethics Committee on Animal Testing, for the RE-Place platform and for the employment of a communications officer. These projects came about under Bianca Debaets' cabinet and continued when Minister Bernard Clerfayt became responsible for animal welfare in the Brussels-Capital Region.
Minister of animal welfare Bernard Clerfayt
Bernard Clerfayt is minister in the Brussels-Capital Region and is responsible, among other matters, for the Animal Welfare Department. In this capacity, he has taken several initiatives to reduce the use of laboratory animals. For example, he supports the in vitro projects carried out within the Toxicology Department of the Vrije Universiteit Brussel. Those projects offer young researchers the opportunity to gain experience and expertise in the development and use of human stem cell cultures to study the side effects of drugs in the human liver. In addition, the Brusselsgovernment explicitly supports the RE-Place database, a joint project of Flanders and Brussels to centralise in a database all animal replacement methods for which expertise exists in Belgium. This will allow young researchers easy access to new technologies and to build up experience in the use of non-animal methods.
Minister Clerfayt vigorously supports IC-3Rs initiatives and officially expressed this support during his welcome speeches at the Symposia organised by IC-3Rs. The 2022 IC-3Rs Symposium, titled "More science, more care, fewer animals", and the 2021 online edition on animal testing in drug development were opened in this manner by the minister.
During the study days organised by the Brussels-Capital Region, IC-3Rs was positively highlighted. On Monday 27 March 2023, IC-3Rs took part in an interesting study day on animal welfare and alternative methods. Fifteen different speakers gave short presentations on the objectives of the scientific organisations they are affiliated with or on their research using non-animal methods. Find the report of the event here.
Activities of the VUB Ethics Committee on Animal Testing
Scientists who use laboratory animals need continuous training to deliver high-quality and ethical research in the context of the gradual reduction in the number of laboratory animals and the increasing interest in the welfare of laboratory animals. Therefore, with the financial support of the Brussels-Capital Region, IC-3Rs enables projects within the VUB related to the 3Rs.
For example, the Animal Ethics Committee organises several workshops within the framework of the refinement principle. During one of the workshops, the focus was on the assessment and scores assigned with regard to the severity (discomfort, pain, ....) of animal experiments. Complementing the workshop, the Ethics Committee, in collaboration with the Animalarium and the Animal Welfare Body, prepared a guidance document indicating the degree of pain for different disease models and procedures in laboratory animals. The document provides researchers with a clear frame of reference for how certain procedures affect the welfare of laboratory animals and is therefore a useful tool to prevent unnecessary suffering. View the guidance document for prospective severity assessments of animal experiments here.
Doctoral Theses
The Brussels Capital Region sponsored part of the PhD theses
Running research
Sponsorship during 2021-2022: running PhD thesis: Alexandra Gatzios
Non-alcoholic fatty liver disease has grown to pandemic proportions, affecting approximately 25% of the general population which makes it the number one chronic liver disease today. Within the next decade, NAFLD is also projected to become the leading cause for liver transplantation. Yet, no pharmaceutical therapies exist for the treatment of NAFLD.
Recently, the nomenclature for NAFLD was revised and the novel terminology of metabolic dysfunction-associated steatotic liver disease (MASLD) was adopted. This change was sparked by improved understanding of the pathogenesis, which is likely much more heterogenic than previously assumed, along with decades of clinical trial failures. The novel nomenclature envisions clinical trial redesign using stratified patient groups based on etiology, with the predominant disease drivers being metabolic-, genetic- or environmentally related, rather than inclusion based on histology score.
Anticipating clinical trial redesign, this research envisions to adopt patient heterogeneity in translational and preclinical research through development of a human-relevant, etiology-based anti-MASLD in vitro drug discovery platform that will include the three main pillars of patient heterogeneity (metabolic, genetic and environmental). The platform will target thyromimetic compounds, as this pathway was found to be dysregulated in MASLD patients and seems to be a promising approach to find new anti-MASLD therapies.
Completed research
Completed and partly sponsored PhD thesis: 2017-2020: Joost Boeckmans
Development and characterization of a human stem cell-based in vitro model for anti-NASH drug testing
Non-alcoholic steatohepatitis (NASH) is a severe chronic liver disease that affects about 5% of the population. NASH is characterized by hepatic lipid accumulation, inflammation and fibrosis and can progress to cirrhosis and hepatocellular carcinoma. There are currently no drugs available to treat NASH. Investigation of NASH traditionally relies on animal models, which are often not representative for the human situation. Therefore, the aim of the doctoral thesis was to develop a human-based in vitro model that can recapitulate the molecular and cellular mechanisms that drive NASH and can be used during anti-NASH drug development. The project was supervised by Prof. Robim M Rodrigues, Prof. Tamara Vanhaecke, Prof. Vera Rogiers and Prof. Joery De Kock from the Vrije Universiteit Brussel.
Completed and partly sponsored PhD thesis: 2017-2020: Alessandra Natale
Shift from 2D to 3D in the development of a human skin cell-derived hepatic in vitro model for toxicological applications
Drug-induced liver injury (DILI) is a major threat to human health and is, together with absence of clinical efficiency, at the basis of a 90% failure rate in drug development. As preclinical data obtained using experimental animals do not adequately represent the human situation, in vitro models based on human cells are highly needed. In this doctoral thesis, the relevance of human-skin derived stem cells differentiated towards hepatic cells (hSKP-HPC) for toxicological screening was highlighted and the biological importance of ‘in vivo-like’ conditions for culturing and differentiating human stem cells was emphasized. Although the process requires further optimization, the results pave the way to enhance hepatic maturation which is a prerequisite to reliably predict human-specific hepatotoxicity of new pharmaceuticals. The project was supervised by Prof. Robim M. Rodrigues, Prof. Tamara Vanhaecke, Prof. Vera Rogiers and Prof. Joery De Kock from the Vrije Universiteit Brussel.
Vision on the Use of Test Animals
The medical scientific mission of the Vrije Universiteit Brussel (VUB) entails that, to fulfill its social responsibility, it conducts innovative research aimed at preventing, predicting and curing diseases, among other things.
In doing so, the VUB strives for an optimal research environment by providing the necessary support for researchers and by enabling and requiring continuous training. Moreover, every VUB researcher signs the "charter of the good researcher" that includes clear guidelines on correct scientific-ethical behaviour as well as the regulations on scientific integrity violations. All this means that research is carried out in a strictly regulated framework. When conducting scientific research at the VUB, not only legal obligations are examined, but also social-ethical considerations. Thus, every researcher must ask himself the question "What is the most appropriate model to answer my research question?" beforehand. Examples of appropriate models are a computer simulation, performing tests on cells and tissues of laboratory animals and/or humans (in vitro research) or performing experiments on laboratory animals (in vivo research). The VUB is strongly committed to the development of non-animal test methods through the expansion of the IC-3R Centre. The Innovation Centre for 3R Alternatives (IC-3Rs) was founded at the VUB on 25 September 2017. This platform aims to stimulate the development, visibility and use of alternative 3R methods (Replacement, Reduction, Refinement) - and to strengthen the communication on this subject, building on the extensive expertise of the IVTD (In VitroToxicology and Dermato- Cosmetology) group. Furthermore stimulate the development of in vitro methods by building on the long-standing activities and expertise of the IVTD research group. Growth is possible thanks to the VUB Chair/legate Mireille Aerens for the development of non-animal test methods and the support of the Brussels Region (Minister Bernard Clerfayt). The focus is on giving opportunities to young researchers and innovative projects to conduct research in this field and thus broaden the basis for non-animal research. IC-3Rs aims to use fewer animals wherever scientifically possible and to increase the focus on integrating alternative non-animal methods into basic and applied research, the areas where most animals are used worldwide.
"Can we develop new medicines without animals today?" All experts clearly agree: today this is not yet possible, but we can carry out parts of the research without using animals among others by using sophisticated cultures of human stem cells...Should animal testing be necessary within a research project, those involved must adhere to the legal and ethical standards set out in (inter)national laws and regulations. These state, among other things, that any project which requires the use of laboratory animals must receive ethical approval from a legally recognised Experimental Ethics Committee (ECD) before it can begin.An important question which the Ethical Committee on Animal Experiments of the VUB always asks itself during the evaluation of a research project is: "What is the social and scientific added value of the expected results of the research and does this added value outweigh the discomfort the test animal might experience?".
If the research on animals has an important added value, we will always strive to apply the legal principles of the 3Rs as much as possible. On the one hand, this means that researchers always use the lowest possible animal species and keep the number of animals to a scientifically justified minimum. On the other hand, the animals must be housed in the best possible conditions and their welfare must be monitored by the researchers, the veterinarians experts and the members of the Animal Welfare Unit. Today, the "best science" is a well-considered combination of in vivo and in vitro methodology, and the follow-up of new developments is crucial so that non-animal techniques can be integrated as soon as they are well established and available. With the construction of a new VUB animalarium, considerable investments have been made in recent years that allow for further optimised housing and animal care. The 3Rs are further complemented by the VUB with the principles of "Commitment" and "Accountability". Every researcher has the obligation to follow training and education and every researcher has to give account to both official bodies and the general public. One of the ways in which the VUB is replacing or reducing the use of laboratory animals is by promoting the use of RE-Place among researchers. This database, supported by Animal Welfare Flanders and the Brussels Region, provides a reliable overview of "New Approach Methodologies (NAMs)", which reduce or even eliminate the need for laboratory animals. In addition, the tool can be used to identify experts and research centres where the techniques can be taught..
At the VUB, there are also several European research projects that endorse the 3Rs, such as Ontox, Twinalt, PARC...
Furthermore, the VUB signed the transparency agreement on animal testing in Belgium. The aim of this Agreement is to ensure that members of the Belgian public receive accurate and up-to-date information on animal testing:
- how such research is regulated in Belgium
- the role it plays in the overall process of scientific discovery, treatment development and safety testing
- the efforts made by researchers and staff to support animal care and welfare
- what is being done to reduce the use of animals and minimise their suffering.






